Reactions of OH and NO radicals with 1,1-dichloroethylene in argon matrices. FTIR and theoretical studies
Abstract
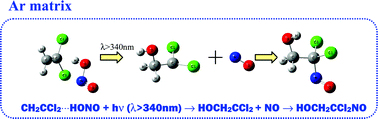
HONO/1,1-dichloroethylene/Ar matrices were subjected to UV radiation (λ > 340 nm) from a medium pressure mercury lamp. The products of the photolysis were studied experimentally by means of FTIR spectroscopy and theoretically using the ab initio MP2 method. Two conformers of 2-nitroso-2,2-dichloroethanol molecule have been identified as the final products of the double addition reaction of the OH, NO radicals to 1,1-dichloroethylene. The additional reactive species observed in the matrix is tentatively identified as an 1,1-dichloro-2-hydroxyethyl radical, an intermediate formed by single addition of OH to 1,1-dichloroethylene. The three photoproducts have been identified and observed for the first time. The identities of the products have been justified by comparison with the experiments with deuterated DONO and by performing concentration and annealing studies as well as by reference to the spectral data of related molecules. The results of the quantum mechanical calculations confirmed both the assignment of the new molecules and mechanism of the reaction observed in our experiment.

 Please wait while we load your content...
Please wait while we load your content...