Kinetic study of the reactions of the sodium dimer (Na2) with a range of atmospheric species
Abstract
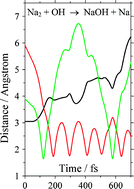
The reactions of Na2 with a series of atmospheric constituents were studied using a fast flow tube with detection of Na2 by laser induced fluorescence at 656.2 nm [Na2(A1Σ+u − X1Σ+g)]. The resulting rate coefficients at 298 K for the reactions of Na2 with OH, O2, NO2, NO, O3, H, H2 and H2O are: (1.01+0.35−0.25) × 10−10, (2.95 ± 0.46) × 10−11, (1.79+0.51−0.31) × 10−10, (1.33 ± 0.16) × 10−11, (8.0+24−3.0) × 10−11, ≤6 × 10−12, ≤4 × 10−15, and ≤3 × 10−13 cm3 molecule−1 s−1, respectively. The quoted uncertainties include measurement imprecision at the 1σ level, and systematic errors. The reaction between Na2 and OH produces chemiluminescence at 589 nm [Na(32PJ – 32S1/2)], with a measured branching ratio of (7.6+15.0−3.7) × 10−3. The reaction enthalpies are calculated using quantum theory at the Complete Basis Set (CBS-Q) level; all reactions except Na2 + H2O and Na2 + H2 are exothermic. The surprisingly slow reaction of Na2 with OH is explained using trajectory calculations and consideration of the splitting between the covalent and ionic surfaces involved in the reaction, coupled with the Landau–Zener formalism. The small upper limit to the rate coefficient for the strongly exothermic reaction Na2 + H appears to be a striking example of the light atom anomaly where the reaction is kinematically constrained.

 Please wait while we load your content...
Please wait while we load your content...