Probing the validity of the Derjaguin approximation for heterogeneous colloidal particles
Abstract
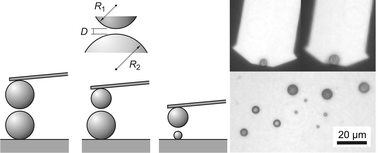
The Derjaguin approximation states that the interaction force between two curved surfaces is proportional to their effective radius, whereby the inverse effective radius is the arithmetic mean of the inverse curvature radii of the surfaces involved. The present study investigates the validity of this approximation with an atomic force microscope (AFM) by measuring interaction forces between colloidal particles of different sizes, but of identical composition. Forces were measured between silica particles of 2.0, 4.8 and 6.8 μm in diameter in KCl electrolyte solution with and without adsorbed poly(amido amine) (PAMAM) dendrimers. The Derjaguin approximation could be confirmed at all distances investigated, including those comparable with the characteristic length scales of the surface roughness or the surface charge heterogeneities. For the conditions investigated, the Derjaguin approximation turns out to be surprisingly robust.

 Please wait while we load your content...
Please wait while we load your content...