Deactivation of the first excited singlet state of thiophenols
Abstract
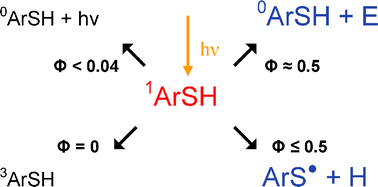
On the bases of picosecond and nanosecond laser flash photolysis with detection by emission and absorption spectroscopy, a quantitative description is given of all deactivation channels of the first excited singlet state of thiophenols ArSH(S1) such as fluorescence, intersystem crossing (ISC), chemical dissociation into radicals, and radiation-less internal conversion (IC). For this purpose, the photolysis of thiophenol and its methyl-, methoxy-, and chloro-substituted derivatives was studied in solvents of increasing polarity: 1-chlorobutane, ethanol, and acetonitrile. The fluorescence lifetime of the thiophenols was found to range from some hundreds of picoseconds up to a few nanoseconds, correlating with fluorescence quantum yields between 0.001–0.040, at room temperature. Depending on the substitution pattern of the aromatic ring, the quantum yield of the S–H bond dissociation was found to be between 0.3–0.5, irrespective of the solvent polarity. In laser photolysis, no triplet formation of the investigated compounds could be observed neither by the direct way nor by subsequent sensitization with β-carotene. As a difference to the total, the radiation-less internal conversion (ΦIC ≥ 0.5) was found to be the dominating process.

 Please wait while we load your content...
Please wait while we load your content...