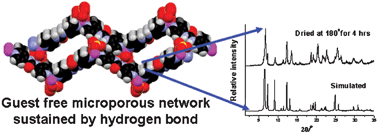
Effects of hydrogen bonding backbone, ligating topology and counter-anions on the resultant framework topologies of a series of metal–organic frameworks (MOFs) derived from two hydrogen bond functionalized ligands, namely N,N′-bis(4-pyridyl)urea L1 and N,N′-bis(3-pyridyl)urea L2 (which are positional isomers) and Zn(II) salts having different counter-anions such as chloride, acetate and sulfate have been studied using single crystal X-ray diffraction techniques. The counter-anions seem to play major role in controlling the network topologies in the MOFs derived from L1 having linear ligating topology whereas the flexible ligating topology of the ligand appears to have much influence on the resultant networks of the MOFs derived from L2. The complementary hydrogen bonding interactions of the urea backbone is not observed in any of these MOFs. However, urea functionality efficiently binds the counter-anions/solvents in these structures and contributes significantly to shaping the hierarchical supramolecular structure formation. The structures of [{Zn(L1)(SO4)}·EG·3H2O]n (3) and [{Zn(L2)(Cl)2]n (4) are especially interesting. While 3 showed robust microporous architecture even after removal of guest molecules as revealed by XRPD data, 4 showed 2-D network of left-handed helix sustained by N–H⋯Cl interactions. The microporosity of 3 has been studied by N2 gas sorption
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...