An enantioselective desymmetrisation approach to C9-substituted trans-hydrindene rings based on a diastereotopic group-selective intramolecular Diels–Alder reaction†
Abstract
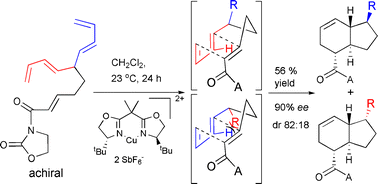
The synthesis of an achiral skipped bis(1,3-diene) substrate was achieved, which was shown to undergo an enantioselective, diastereotopic group-selective, intramolecular Diels–Alder reaction.

 Please wait while we load your content...
Please wait while we load your content...