Fluorous phase-transfer activation of catalysts: application of a new rate-enhancement strategy to alkene metathesis†
Abstract
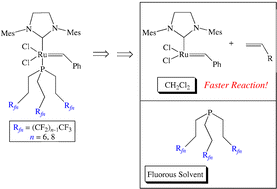
Reactions of the bis(pyridine) complex (H2IMes)(Py)2(Cl)2Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh) and fluorous phosphines P(CH2CH2Rfn)3 (n = a, 6; b, 8; c, 10; Rfn = (CF2)n–1CF3) give (H2IMes)(P(CH2CH2Rfn)3)(Cl)2Ru(
CHPh) and fluorous phosphines P(CH2CH2Rfn)3 (n = a, 6; b, 8; c, 10; Rfn = (CF2)n–1CF3) give (H2IMes)(P(CH2CH2Rfn)3)(Cl)2Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh) (2a–c, 64–73%), which are analogs of Grubbs' second generation
CHPh) (2a–c, 64–73%), which are analogs of Grubbs' second generation

 Please wait while we load your content...
Please wait while we load your content...