Bond length and bond multiplicity: σ-bond prevents short π-bonds†
Abstract
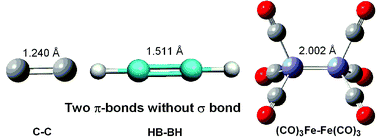
Analysis of model compounds such as Fe2(CO)6, C2 and HBBH shows that π-bonds left to themselves are shorter than σ-bonds; in many ways σ-bonds prevent π-bonds from adopting their optimal shorter distances.

 Please wait while we load your content...
Please wait while we load your content...