Synthesis of novel l-N-MCd4T as a potent anti-HIV agent†
Abstract
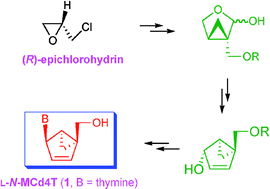
L-N-MCd4T (1) has been synthesized as a potent anti-HIV agent starting from (R)-epichlorohydrin using tandem alkylation, chemoselective reduction of ester in the presence of lactone functional group, RCM reaction and Mitsunobu reaction as key steps and was found to be a very potent anti-HIV-1 (EC50 = 6.76 µg mL−1) agent without cytotoxicity up to 100 µg mL−1, indicating that the anti-HIV-1 activity found is similar to that of ddI (EC50 = 4.95 µg mL−1), which is used clinically for the treatment of AIDS patients.

 Please wait while we load your content...
Please wait while we load your content...