Non-equivalence of d- and l-trehalose in stabilising alkaline phosphatase against freeze-drying and thermal stress. Is chiral recognition involved?
Abstract
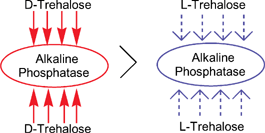
Comparison of the ability of the enantiomeric forms of trehalose to stabilise alkaline phosphatase towards dehydration and heat showed that natural D-trehalose is superior to L-trehalose, although both

 Please wait while we load your content...
Please wait while we load your content...