Synthesis of triester-functionalized molecular motors incorporating bis-acetylide trans-platinum insulating fragments†‡
Abstract
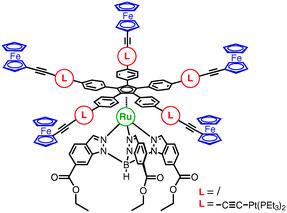
Bis-ferrocene compounds linked either by two triple bonds (1,4-di(ferrocenyl)butadiyne 1), or by the triple bond–platinum–triple bond sequence (trans-bis(ferrocenylethynyl)bis(triethylphosphine)platinum(II), 2) have been synthesized. The electronic coupling between the ferrocene groups has been estimated from the intensity of the intervalence transition in the electrochemically generated mixed valence complexes. Upon insertion of a platinum fragment a weak attenuation was observed, with the Vab parameter decreasing from 0.036 eV for 1 to 0.025 eV for 2. A theoretical study has also been performed, using a combination of DFT for geometry optimization and Extended Hückel Theory for the estimation of the electronic coupling. It was found that the electronic coupling decreases from 0.090 eV for 1 to 0.022 eV for a model of 2. In a second part of this work, we describe the synthesis of two molecular motors incorporating the ligand hydrotris[6-(ethoxycarbonyl)indazol-1-yl]borate which exhibits three pendant ester groups dedicated to be anchored onto an oxide surface. This stator is connected through a ruthenium centre to a pentasubstituted cyclopentadienyl rotor bearing ferrocene terminal electroactive groups, linked either by a phenylethynyl spacer (complex 4) or a spacer containing bis-acetylide trans-platinum insulating fragments (complex 8).

 Please wait while we load your content...
Please wait while we load your content...