Density and viscosity of several pure and water-saturated ionic liquids
Abstract
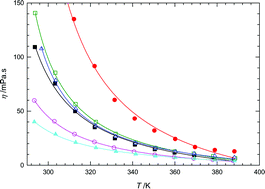
Densities and viscosities were measured as a function of temperature for six ionic liquids (1-butyl-3-methylimidazolium hexafluorophosphate, 1-butyl-3-methylimidazolium tetrafluoroborate, 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, 1-ethyl-3-methylimidazolium ethylsulfate and butyltrimethylammonium bis(trifluoromethylsulfonyl)imide . The density and the viscosity were obtained using a vibrating tube densimeter from Anton Paar and a rheometer from Rheometrics Scientific at temperatures up to 393 K and 388 K with an accuracy of 10−3 g cm−3 and 1%, respectively. The effect of the presence of water on the measured values was also examined by studying both dried and water-saturated samples. A qualitative analysis of the evolution of density and viscosity with cation and anion chemical structures was performed.

 Please wait while we load your content...
Please wait while we load your content...