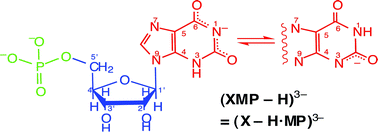
The stability constants of the mixed-ligand complexes formed between Cu(Arm)2+ [Arm = 2,2′-bipyridine (Bpy) or 1,10-phenanthroline (Phen)], and the di- or trianion of xanthosine 5′-monophosphoric acid [= XMP2− or (XMP − H)3−] were determined by potentiometric pH titration in aqueous solution (25 °C; I = 0.1 M, NaNO3). Those for the monoanion, i.e., the Cu(Arm)(H;XMP)+ complexes, could only be estimated; for these species it is concluded that the metal ion is overwhelmingly bound at N7 and the proton resides at the phosphate group. Similarly, in the Cu(Arm)(XMP)± [= Cu(Arm)(X − H·MP·H)±] complexes Cu(Arm)2+ is also at N7 but the xanthine residue has lost a proton whereas the phosphate group still carries one, i.e., stacking plays, if at all, only a very minor role, yet, the N7-bound Cu(Arm)2+ appears to form an outer-sphere macrochelate with P(O)2(OH)−, its formation degree being about 60%. All this is different in the Cu(Arm)(XMP − H)− complexes, which are formed by the (XMP − H)3− species, that occur at the physiological pH of 7.5 and for which previously evidence has been provided that in a tautomeric equilibrium the xanthine moiety loses a proton either from (N1)H or (N3)H. In Cu(Arm)(XMP − H)− the phosphate group is the primary binding site for Cu(Arm)2+ and the observed increased complex stability is mainly due to intramolecular stack (st) formation between the aromatic-ring systems of Phen or Bpy and the monodeprotonated xanthine residue of (XMP − H)3−; e.g., the stacked Cu(Phen)(XMP − H)−st isomer occurs with approximately 76%. Regarding biological systems the most important result is that at physiological pH the xanthine moiety has lost a proton from the (N1)H/(N3)H sites forming (XMP − H)3− and that its anionic xanthinate residue is able to undergo aromatic-ring stacking.

 Please wait while we load your content...
Please wait while we load your content...