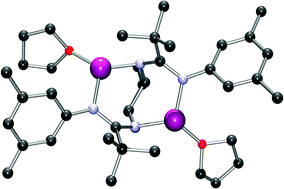
A series of racemic N-aryl-substituted trans-1,2-diaminocyclohexyl (t-1,2-DACH)-linked bis(amidines) were synthesised and their solution behaviour, and solid-state structures were investigated. The amidine functionalities within these compounds were extremely sterically hindered, with ortho-aryl substitution found to hinder N–Ar bond rotation. The results of these studies were used to rationalise the lack of reactivity of these compounds with [Y{N(SiMe3)2}3]. Dilithiation of the t-1,2-DACH linked bis(amidines) did, however, proceed easily and the solution behaviour and solid-state structures of the resulting THF-solvated lithium amidinates were investigated. All the compounds showed similar structures in the solid-state, while NMR experiments indicated that the solid-state structures were likely to be maintained in solution. Attempted metathesis reactions with YCl3 did not, however, yield the desired yttrium chloride complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...