Kinetics of the light-driven aqueous autoxidation of sulfur(iv) in the absence and presence of iron(ii)†
Abstract
The photochemical autoxidation of aqueous, acidic sulfur(IV) solutions was studied in the absence and presence of iron(II) by a newly introduced technique using a diode-array spectrophotometer, in which the same light source is used to drive and detect the reaction. Based on detailed kinetic and stoichiometric data sets, a non-chain mechanism is proposed for the autoxidation of sulfur(IV). In this mechanism, excited hydrated sulfur dioxide, *H2O·SO2, first reacts with O2 to form peroxomonosulfate ion, HSO5−, which rapidly oxidizes another H2O·SO2 to give hydrogensulfate ion as a final product. In the presence of iron(II), the formation of iron(III) was detected, which can be interpreted through the simultaneous contribution of two additional pathways: some of the HSO5− formed oxidizes iron(II) instead of sulfur(IV), and *H2O·SO2 also reacts directly with iron(II) to yield iron(III). This mechanism provides a sufficient quantitative interpretation of all experimental observations.
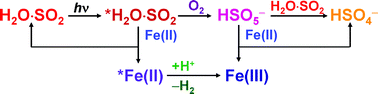

 Please wait while we load your content...
Please wait while we load your content...