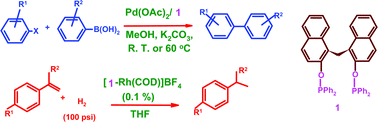
Transition metal complexes of bis(2-diphenylphosphinoxynaphthalen-1-yl)methane (1) are described. Bis(phosphinite) 1 reacts with Group 6 metal carbonyls, [Rh(CO)2Cl]2, anhydrous NiCl2, [Pd(C3H5)Cl]2/AgBF4 and Pt(COD)I2 to give the corresponding 10-membered chelate complexes 2, 3 and 5–8. Reaction of 1 with [Rh(COD)Cl]2 in the presence of AgBF4 affords a cationic complex, [Rh(COD){Ph2P(–OC10H6)(µ-CH2)(C10H6O–)PPh2-κP,κP}]BF4 (4). Treatment of 1 with AuCl(SMe2) gives mononuclear chelate complex, [(AuCl){Ph2P(–OC10H6)(µ-CH2)(C10H6O–)PPh2-κP,κP}] (9) as well as a binuclear complex, [Au(Cl){µ-Ph2P(–OC10H6)(µ-CH2)(C10H6O–)PPh2-κP,κP}AuCl] (10) with ligand 1 exhibiting both chelating and bridged bidentate modes of coordination respectively. The molecular structures of 2, 6, 7, 9 and 10 are determined by X-ray studies. The mixture of Pd(OAc)2 and 1 effectively catalyzes Suzuki cross-coupling reactions of a range of aryl halides with aryl boronic acid in MeOH at room temperature or at 60 °C, giving generally high yields even under low catalytic loads. The cationic rhodium(I) complex, [Rh(COD){Ph2P(–OC10H6)(µ-CH2)(C10H6O–)PPh2-κP,κP}]BF4 (4) catalyzes the hydrogenation of styrenes to afford the corresponding alkyl benzenes in THF at room temperature or at 70 °C with excellent turnover frequencies.

 Please wait while we load your content...
Please wait while we load your content...