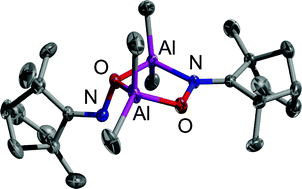
The four organoaluminium ketoximates [(2,4-dimethylpentane-3-one oximato)AlMe2]2 (1), (meso)-[(norcamphor oximato)AlMe2]2 (2), {[(R,R)-camphor oximato]AlMe2}3 (3) and {[(R,S)-fenchone oximato]AlMe2}2 (4) have been prepared by the reactions of the corresponding oximes with trimethylaluminium. All compounds have been fully characterized by means of IR, mass and multi-nuclear NMR spectroscopy (1H, 13C, 27Al) and by elemental analyses. The crystal structures of three of these compounds (2, 3 and 4) were determined, revealing the aggregation motif of organometallic group 13 oximates to vary from the hitherto predominant six-membered dimeric M2N2O2 array to others (six-membered M3O3 core ring in the case of the camphor derivative; five-membered M2NO2 core ring in the case of the fenchone derivative) so far only found e.g. in hydroxylamino complexes of group 13 metals. Furthermore, complexes 3 and 4 exhibit an unusual behaviour in solution, as indicated by upfield-shifted additional signals in their 27Al NMR spectra, pointing at aluminium atoms in penta- or hexacoordination, respectively.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...