Chiral recognition between lactic acid derivatives and an aromatic alcohol in a supersonic expansion: electronic and vibrational spectroscopy
Abstract
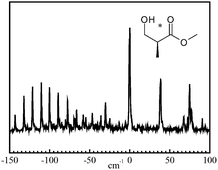
Jet-cooled diastereoisomeric complexes formed between a chiral probe, (±)-2-naphthyl-1-ethanol, and chiral lactic acid derivatives have been characterised by laser-induced fluorescence and

 Please wait while we load your content...
Please wait while we load your content...