Comparative study of cluster- and supercell-approaches for investigating heterogeneous catalysis by electronic structure methods: Tunneling in the reaction N + H → NH on Ru(0001)
Abstract
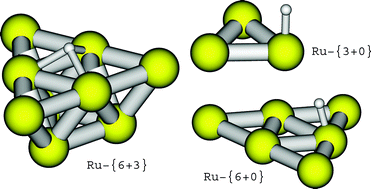
Different ruthenium clusters of various sizes are constructed with the aim to model the Ru(0001) surface with a sufficient accuracy for predicting catalysis by hybrid density functional methods (B3LYP). As an example reaction the hydrogenation step N(ads) + H(ads) → NH(ads) from the catalytic production cycle of

 Please wait while we load your content...
Please wait while we load your content...