Mechanistic insight into copper-catalysed allylic substitutions with bis(triorganosilyl) zincs. Enantiospecific preparation of α-chiral silanes†
Abstract
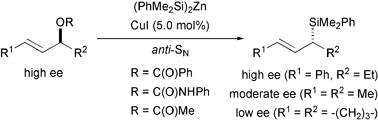
Isotopic desymmetrisation, as well as (stereo)chemical correlation, has illuminated significant aspects of the σ–π–σ mechanism of copper-catalysed allylic substitution reactions: an enantiospecific and regioselective access to α-chiral

 Please wait while we load your content...
Please wait while we load your content...