Synthesis and crystal structure of Li4BH4(NH2)3†
Abstract
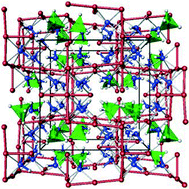
The solid solution, (LiNH2)x(LiBH4)(1−x), formed through the reaction of the two potential hydrogen storage materials, LiNH2 and LiBH4, is dominated by a compound that has an ideal stoichiometry of Li4BN3H10 and forms a body-centred cubic structure with a lattice constant of ca. 10.66 Å.

 Please wait while we load your content...
Please wait while we load your content...