Facile preparation of the oxetane-nucleosides†
Abstract
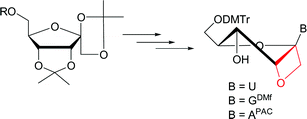
Efficient and practical large scale synthesis of suitably protected 1′,2′-oxetane locked purine and pyrimidine nucleosides for incorporation in oligo-DNA or -RNA by solid-phase synthesis is reported. A high regio and stereoselectivity with preferential formation of the β-anomer in the glycosylation reaction, using the Vorbrüggen procedure, was achieved by a convergent synthetic procedure with orthogonal protection strategy using either 1,2-di-O-acetyl-3,4-O-isopropylidene-6-O-(4-toluoyl)-D-psicofuranose or 2-O-acetyl-6-O-benzyl-1,3,4-tri-O-(4-toluoyl)-D-psicofuranose as the glycosyl donor.

 Please wait while we load your content...
Please wait while we load your content...