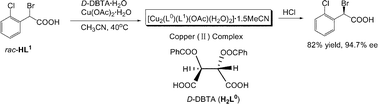
Herein we present a new example of coordination-mediated resolution of racemic acids by a chiral acid. The reaction of copper(II) acetate monohydrate, optically pure O,O′-dibenzoyltartaric acid (DBTA) and racemic α-bromo-2-chlorophenylacetic acid (HL1) in acetonitrile solution afforded a binuclear copper(II) complex with D-DBTA dianion, α-bromo-2-chlorophenylacetate and acetate as ligands. After decomposition of the complex with acid, the optically active acid ((R)–HL1) was obtained. Similarly, α-bromo-2-fluorophenylacetic acid (HL2), α-bromo-2-bromophenylacetic acid (HL3), α-chloro-2-chlorophenylacetic acid (HL4), α-chloro-2-fluorophenylacetic acid (HL5), α-bromophenylacetic acid (HL6), α-bromo-4-chlorophenylacetic acid (HL7), 2-bromopropionic acid (HL8) and 2-chloropropionic acid (HL9) were resolved by the same method. Satisfactory results were obtained for HL2 to HL5. For HL6 and HL7, only racemic acids were obtained. For the two α-halo aliphatic acids (HL8 and HL9), poor enantioselectivity was obtained. It is more interesting that three acids (HL1, HL2 and HL3) could spontaneously racemize in acetonitrile solution, which resulted in crystallization-induced dynamic resolution (CIDR) with greater than 50% yield.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...