Total synthesis of milbemycins: a synthesis of (6R)-6-hydroxy-3,4-dihydromilbemycin E
Abstract
Following studies using benzyloxymethyl isopropenyl ketone 5 and ethyl 3-(3-furyl)-3-oxopropanoate 6, Robinson reactions between aryloxymethyl isopropenyl ketones 19 and 5 and ethyl 3-(2-trimethylsilyl-3-furyl)-3-oxopropanoate 20 were found to be stereoselective giving cyclohexanones 21 and 41, in which the 3-(arylmethoxy) substituents were cis to the 2-hydroxyl groups, as the major products. After reduction and protection of ketone 21, selective PMB-deprotection, oxidation and stereoselective reduction inverted the configuration at C(3) to give the diol 30. Protection of the secondary 3-hydroxyl group followed by modification of the protected 4-alcohol then gave the hydroxybutenolides 36 and 37 after oxidation of the silylated furan using singlet oxygen. The 3-benzyloxycyclohexanone 41 was also converted into the hydroxybutenolide 37via the (2-trimethylsilylethoxy)methyl (SEM) ether 35.
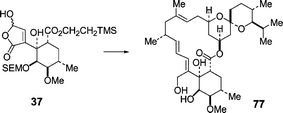
The Wittig reaction between the ylid generated from 2-methylpropyl(triphenyl)phosphonium salt and hydroxybutenolide 36 gave predominantly the (2Z,4Z)-dienyl acid 38 which was taken through to the butenolide 40. Similarly, the racemic hydroxybutenolide 37 was condensed with the racemic ylid derived from phosphonium salt 53 to give, after SEM-deprotection and 5-membered lactone formation, a mixture of the (9Z,2′Z)-dienyl lactones 58 and 59 containing ca. 10% of the corresponding (9Z,2′E)-isomers 60 and 61. (2′Z)/(2′E)-Isomerisation of the dienes 58 and 59 using iodine followed by deprotection gave a mixture of the seco-acids 62 and 63. Selective macrocyclisation of the seco-acid 62 in which the relative configuration of the C(1)–C(7) and C(17)–C(19) fragments (milbemycin numbering) corresponded to that present in the natural milbemycins, gave the β-milbemycin analogue 65 after butenolide reduction. The hydroxybutenolide 37 was also condensed with the ylid derived from the phosphonium salt 1 and the product taken through to (6R)-6-hydroxy-3,4-dihydromilbemycin E 77.
Preliminary attempts to convert the β-milbemycin analogues 65 and 77 into tetrahydrofurans corresponding to analogues of α-milbemycins by treatment with toluene p-sulfonyl chloride under basic conditions gave the primary allylic chlorides 78 and 79.

 Please wait while we load your content...
Please wait while we load your content...