A versatile catalyst for asymmetric reactions of carbonyl groups working purely by activation through hydrogen bonding: Mukaiyama-aldol, hetero Diels–Alder and Friedel–Crafts reactions
Abstract
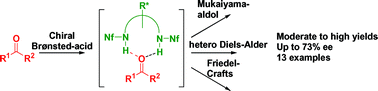
Bis-sulfonamides are demonstrated to be promising candidates for the efficient activation of carbonyl compounds through hydrogen bonding. Exemplified by three carbonyl addition reactions: Mukaiyama-aldol, hetero Diels–Alder and Friedel–Crafts reactions we show that bis-triflamides or bis-nonaflamides of commercially available chiral diamines act as chiral Brønsted-acid catalysts, leading to the optically active products in moderate to excellent yields and with enantioselectivities up to 73% ee.

 Please wait while we load your content...
Please wait while we load your content...