A comparative analysis of the total syntheses of the amphidinolide T natural products
Abstract
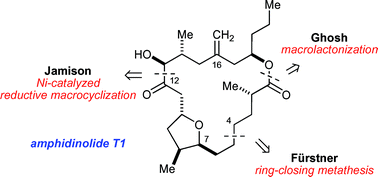
In this article we compare and contrast the strategies and tactics used in the syntheses of the amphidinolide T family of natural products that have been reported by Fürstner, Ghosh and ourselves. Similar approaches to the trisubstituted THF ring present in the targets are utilized in all of the syntheses, but each strategy showcases a different means of macrocyclization.

 Please wait while we load your content...
Please wait while we load your content...