Iodo- and bromo-enolcyclization of 2-(2-propenyl)cyclohexanediones and 2-(2-propenyl)cyclohexenone derivatives using iodine in methanol and pyridinium hydrobromide perbromide in dichloromethane
Abstract
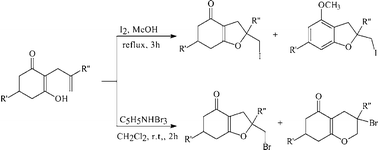
α-Allylcyclohexane-1,3-diones undergo one-pot iodine–

 Please wait while we load your content...
Please wait while we load your content...