Synthesis of 3,4-dihydro-2H-pyrans by hetero-Diels–Alder reactions of functionalized α,β-unsaturated carbonyl compounds with N-vinyl-2-oxazolidinone
Abstract
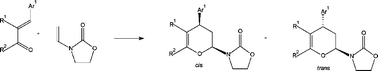
Cycloadditions of 3-aryl-2-benzoyl-2-propenenitriles 1a–d and 3-phenylsulfonyl-3-buten-2-one 1e to N-vinyl-2-oxazolidinone 2 proceed regio- and diastereoselectively yielding cis and trans diastereoisomers of 4-aryl-3,4-dihydro-2-(2-oxo-3-oxazolidinyl)-2H-pyrans 3a–e in 37–65% yield. Cycloadducts cis-3 were the major products. Reaction of 5-arylidene-1,3-dimethylbarbituric acids 4a–c with dienophile 2 afforded mixtures of 2H-pyrano[2,3-d]pyrimidine-2,4(3H)-diones trans5a–c and products 6a–c resulted from an elimination of

 Please wait while we load your content...
Please wait while we load your content...