On the utility of the azido transfer protocol: synthesis of 2- and 5-azido N-methylimidazoles, 1,3-thiazoles and N-methylpyrazole and their conversion to triazole–azole bisheteroaryls†
Abstract
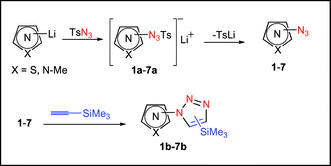
The azido transfer procedure of heteroaryllithium and tosyl azide was used to synthesize selected 2- and 5-azidoazoles. This procedure, which is based on the fragmentation of the appropriate lithium triazene salts 1a–7a, successfully afforded 2-azido-N-methylimidazole 1, 2-azido-1,3-thiazole 2, 2-azidobenzo-1,3-thiazole 3, 5-azido-N-methylpyrazole 4, 5-azido-N-methylimidazole 6 [via 2-(trimethylsilyl)-5-azido-N-methylimidazole 5], and 5-azido-1,3-thiazole 7 (via 5-lithio-1,3-thiazole), but attempts to prepare 5-azido-2-(trimethylsilyl)-1,3-thiazole 8 from the corresponding triazene 7a failed, affording only the desilylated azide 7 in poor yield. Azides 1–7 underwent 1,3-dipolar cycloaddition when mixed with neat (trimethylsilyl)acetylene, giving 1-heteroaryl-4-trimethylsilyl-1,2,3-triazoles 1b–7b generally in very high yields.

 Please wait while we load your content...
Please wait while we load your content...