From a saturated C6H6 solution of racemic 4-(tetrahydro-4H-thiopyran-1-oxide-4-ylidene)-cyclohexanone oxime [1
(1-R/1-S)] the co-crystal (1)4444·C6666H6666 is crystallized. Single crystal X-ray analysis showed that (1)4444·C6666H6666
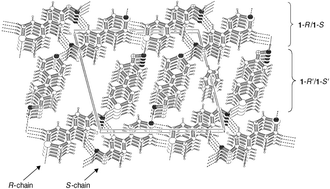
(P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group) in the solid-state consists of enantiomorphous, non-covalent polymer-like chains that contain, in an alternating fashion, the crystallographically independent enantiomers 1-R and 1-R′ or 1-S and 1-S′, respectively. Within each chain the enantiomers are linked by ‘head-to-tail’ intermolecular oxime–sulfoxide hydrogen bonding [D(2) motif]. Neighbouring chains consist of enantiomers with opposite configuration and possess opposite molecular ‘head-to-tail’ alignments. The enantiomorphous chains are interconnected by weak intermolecular C–H⋯O hydrogen bonds involving centrosymmetric C–H⋯oxime [R22(12)] and C–H⋯sulfoxide [R22(8)] motifs between the 1-R and 1-S molecules in neighbouring chains; a nearly planar two-dimensional hydrogen bonding network motif is obtained. In the crystallographic direction [1 0 0] the layers stack in such a fashion that chains occupying successive layers with an identical ‘head-to-tail’ alignment are positioned on top of each other. Concomitantly, channels with areas of ca. 25 Å2 are obtained, which are occupied by C6H6 solvent molecules. A comparison of the IR and Raman spectra of (1)4444·C6666H6666 with those obtained for native 1 that does not contain C6H6, indicates that intermolecular oxime–sulfoxide hydrogen bonding [D(2) motif] also occurs for native 1 in the solid-state.
space group) in the solid-state consists of enantiomorphous, non-covalent polymer-like chains that contain, in an alternating fashion, the crystallographically independent enantiomers 1-R and 1-R′ or 1-S and 1-S′, respectively. Within each chain the enantiomers are linked by ‘head-to-tail’ intermolecular oxime–sulfoxide hydrogen bonding [D(2) motif]. Neighbouring chains consist of enantiomers with opposite configuration and possess opposite molecular ‘head-to-tail’ alignments. The enantiomorphous chains are interconnected by weak intermolecular C–H⋯O hydrogen bonds involving centrosymmetric C–H⋯oxime [R22(12)] and C–H⋯sulfoxide [R22(8)] motifs between the 1-R and 1-S molecules in neighbouring chains; a nearly planar two-dimensional hydrogen bonding network motif is obtained. In the crystallographic direction [1 0 0] the layers stack in such a fashion that chains occupying successive layers with an identical ‘head-to-tail’ alignment are positioned on top of each other. Concomitantly, channels with areas of ca. 25 Å2 are obtained, which are occupied by C6H6 solvent molecules. A comparison of the IR and Raman spectra of (1)4444·C6666H6666 with those obtained for native 1 that does not contain C6H6, indicates that intermolecular oxime–sulfoxide hydrogen bonding [D(2) motif] also occurs for native 1 in the solid-state.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group) in the solid-state consists of enantiomorphous, non-covalent
space group) in the solid-state consists of enantiomorphous, non-covalent 

 Please wait while we load your content...
Please wait while we load your content...