Persistent π radical cations: self-association and its steric control in the condensed phase
Abstract
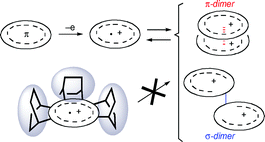
π Radical cations, which are highly reactive in general, can be made persistently stable by appropriate structural modification with heteroatoms, π-conjugated systems, and alkyl substituents. Many of these π radical cations undergo self-association in the condensed phase. The steric control of such self-association of stabilized π radical cations is the subject of the present article. Such an association can result in the formation of π- and/or σ-dimers. The π-dimerization in particular is now considered as an important intermolecular interaction for model studies of a charge-transport phenomenon in positively doped conducting polymers. On the other hand, the intermolecular interactions can be suppressed when the π-system is modified with sterically demanding structural units, for example, by annelation with bicycloalkene frameworks. This structural modification not only brings about unusual stabilization of the radical cations but provides valuable information on the electronic structure/properties of the positively charged π-systems in a segregated state.

 Please wait while we load your content...
Please wait while we load your content...