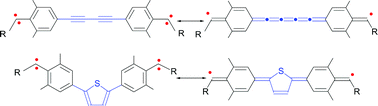
(2,6-Dimethyl-4-tert-butylphenyl)(2,4,6-tribromophenyl)diazomethane(1-N2) was found to be stable enough to survive under Sonogashira coupling reaction conditions, and aryldiazomethyl substituents were introduced at the 1,4-positions of butadiyne (4-2N2) and the 2,5-positions of thiophene(5-2N2). Irradiation of those bis(diazo) compounds generated bis(carbenes), which were characterized by using ESR and UV/vis spectroscopic techniques in a matrix at low temperature as well as time-resolved UV/vis spectroscopy in solution at room temperature. These studies revealed that both of the bis(carbenes), 4 and 5, have singlet quinoidal diradical ground states with a very small singlet–triplet energy gap of less than 1 kcal mol−1. A remarkable increase in the lifetime of bis(carbenes), as opposed to that of the monocarbene (2), was noted and was interpreted to indicate that bis(carbenes) are thermodynamically stabilized as a result of delocalization of unpaired electrons throughout the π net framework. In spite of the stability, both bis(carbenes) are readily trapped by molecular oxygen to afford bis(ketones). Presumably, the reaction of the upper-lying localized quintet states with oxygen is much faster than that for lower-lying states.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...