Diels–Alder reaction of highly substituted 2H-pyran-2-ones with alkynes: reactivity and regioselectivity†
Abstract
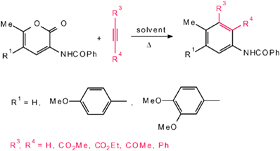
The Diels–Alder reactions of a variety of electron-rich 2H-pyran-2-ones 4 with alkynes 2 yielding aniline derivatives 6 under thermal conditions as well as at high pressures are presented. The effects of the substituents at the positions 3 and 5 in the 2H-pyran-2-one ring on the reactivity and the regioselectivity with different alkynes were qualitatively explained on the basis of electron demand and also by the formation of zwitterionic intermediates.

 Please wait while we load your content...
Please wait while we load your content...