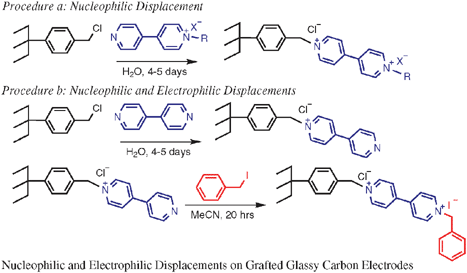
4,4′-Bipyridinium (i.e., viologen) was immobilized on 4-(chloromethyl)phenyl grafted glassy carbon electrodes by a nucleophilic substitution reaction involving 1-ethyl- or 1-benzyl-4-(4′-pyridyl)pyridinium. Reaction times of about 5 days were required for these surface-constrained processes to go to completion in aqueous solution at room temperature. The applicability of the described procedure was demonstrated by performing the equivalent modification in 2 steps by reacting first with 4,4′-bipyridine, followed by quaternization of the available nitrogen to obtain the viologen functionality, that is, the surface acts as a nucleophile in a substitution reaction. However, the quaternization step was found to be possible for introducing the benzyl group but not the ethyl group. The covalently modified electrodes were reasonably stable to repeated electrochemical sweeping in acetonitrile with a 25% decrease in the observed electroactivity after 100 sweeps at a sweep rate of 2 V s−1. The coverage was determined from the electrochemical response of the viologen moiety to be approximately 3 × 10−10 mol cm−2. In addition to cyclic voltammetry, the presence of viologen was demonstrated by means of X-ray photoelectron spectroscopy and time-of-flight secondary ion mass spectrometry. Scanning images (500 × 500 µm2) obtained by the latter technique indicated that the molecules were distributed uniformly over the entire surface. Scanning tunnelling microscopy was used to follow the individual steps of the modification procedure on highly ordered pyrolytic graphite.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...