Elemental fluorine
Part 18.† Selective direct fluorination of 1,3-ketoesters and 1,3-diketones using gas/liquid microreactor technology‡
Abstract
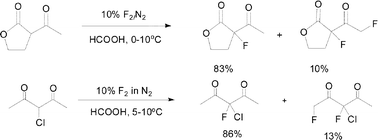
1,3-Ketoesters and 1,3-diketones react with fluorine gas, using Durham multichannel modular microreactor technology, on a preparatively useful scale. High conversions and yields of monofluorinated products were obtained. A consideration of the mechanism of

 Please wait while we load your content...
Please wait while we load your content...