Stoichiometry and doping mechanism of the cubic pyrochlore phase in the system Bi2O3–ZnO–Nb2O5†
Abstract
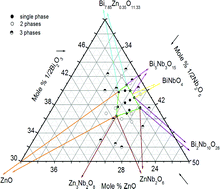
The subsolidus phase diagram of the system Bi2O3–ZnO–Nb2O5 in the region of the cubic pyrochlore phase has been determined at 950 °C. This phase forms a solid solution area that does not include the so-called ideal composition P, Bi3Zn2Nb3O14. It may be described in terms of two compositional variables: ZnO deficiency compared to P together with variable Bi : Nb ratio. Density measurements, preliminary crystallographic studies and the phase diagram indicate the solid solutions to have general formula, Bi3+yZn2−xNb3−yO14−x−y: −0.11(1) ≤ y ≤ 0.14(1) and −0.03(1) ≤ x ≤ 0.31(1).

 Please wait while we load your content...
Please wait while we load your content...