Synthesis of tri-calcium phosphate sponges by interfacial deposition and thermal transformation of self-supporting calcium phosphate films
Abstract
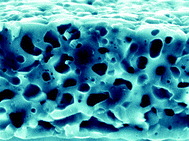
Porous films of β- or α-tri-calcium phosphate (TCP) were prepared by thermal transformation at 1000 °C or 1200 °C, respectively, of thin calcium phosphate/calcium carbonate precursor films precipitated at the air/water interface of a supersaturated carbonic acid solution containing 60 ppm sodium polyaspartate and a [PO43−] : [CO32−] ratio of ca. 2 : 1. The β-Ca3(PO4)2 and α-Ca3(PO4)2 films were typically 10 µm in thickness, consisted of sponge-like interiors with 0.5–1 µm sized macropores, and exhibited surface areas of 10 and 12 m2 g−1 respectively. The thickness and morphology of the TCP flakes were related to the time-dependent structure of the self-supporting composite precursor films, which ranged from highly porous 2-D networks to densely packed agglomerates. Similar films consisting only of calcium phosphate, as well as calcium carbonate films of more complex architecture and up to 150 µm in thickness, were also prepared by polyaspartate-mediated controlled crystallization at the air/water interface. The novel combination of carbonic acid solution and bulk-precipitation inhibitor is described in the preparation of porous minerals that have potential applications as supports for catalysts, for drug delivery, protein adsorption and release, or as bone implant materials.

 Please wait while we load your content...
Please wait while we load your content...