Esterification of carboxylic acids by tributyl borate under solvent- and catalyst-free conditions
Abstract
A new method for esterification of mono- and dicarboxylic acids by tributyl borate has been reported. The esterification reactions have been cleanly carried out in the absence of any solvent under catalyst-free conditions. Boric acid is the only side product, which precipitates during the reaction and can be re-used in the production of tributyl borate. Important mono- and dibutylesters, which have found wide applications as plasticizers and ester base lubricants, were prepared by this manner.
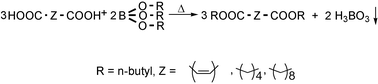

 Please wait while we load your content...
Please wait while we load your content...