The fluoroaryl phosphines P{C6H3(CF3)2-3,5}3
(Laa) and P(C6F5)3
(Lbb) form the complexes trans-[MCl2(Laa)2] and trans-[MCl2(Lbb)2]
(M = Pd or Pt) which have been isolated and fully characterised. 31P NMR studies of competition experiments show that the stability of trans-[PdCl2L2] is in the order L =
Lbb < Laa < PPh3. The crystal structure of trans-[PtCl2(Laa)2] is reported and reveals that the Pt–P bond lengths in trans-[PtCl2L2] are in the order L =
Lbb < Laa < PPh3. The equilibria established when [Pt(norbornene)3] is treated with Laa or Lbb are investigated by 31P and 195Pt NMR spectroscopy and the species [PtLn(norbornene)3−n]
(n
= 1–3) identified. Ligands Laa and Lbb appear to have similar affinities for platinum(0). The complexes trans-[MCl(CO)(Laa)2] and trans-[MCl(CO)(Lbb)2]
(M = Rh or Ir) have been synthesised and fully characterised; the values of νCO are comparable with those for analogous phosphite complexes. The ligands Laa, Lbb, P(C6H2F3-3,4,5)3
(Lccc), P{C6H4(CF3)-2}3
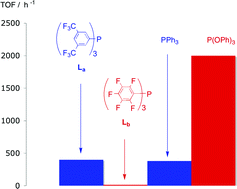
(Ldd), PPh3 and P(OPh)3 have been tested in rhodium-catalysed hydroformylation of 1-hexene and Laa, Lbb, and PPh3 have been tested in rhodium-catalysed hydroformylation of 4-methoxystyrene. Ligands Laa, and Lbb, have been shown to be stable under the hydroformylation catalysis conditions. For the 1-hexene reaction, the activity and selectivity for Laa and Lccc are very similar to the PPh3 catalyst (TOF ca. 400 h−1; n : iso 2.5–3.0) but for the sterically demanding Lbb and Ldd the activity and selectivity was much lower than with PPh3 (TOF ca. 15, n : iso ratio 0.6). Thus, the yield of heptanals obtained with the catalyst derived from Laa is 94% while under the same conditions with Lbb only 6%. The TOF for the Laa/Rh catalyst was 5 times lower than for the P(OPh)3/Rh catalyst despite the superficially similar ligand electronic characteristics for Laa and P(OPh)3.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...