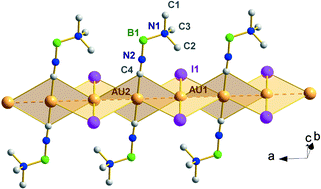
This paper provides evidence that the degree of aurophilic character and resulting solid state arrangement observed in (isocyanide)gold(I) halides is in part dependent on the nature of halide substituent. In particular, we report here the synthesis of a series of novel (isocyanide)gold(I) halides involving trimethylamine-isocyanoborane, an unusual ‘zwitterionic’ isocyanide that is comparable to the more familiar methyl isocyanide, but potentially more strongly Lewis basic. The reaction of chloro(dimethyl sulfide)gold(I) and the isocyanoborane species, (RNC)
(R =
(H3C)3NB(H)2), gives the novel crystalline adduct [(RNC)AuCl]
(1). Treatment of 1 in an organic phase with aqueous KBr effected substitution of the chloride to give the bromide analogue [(RNC)AuBr]
(2). The structures of 1 and 2 were determined by X-ray crystallography to be isostructural, consisting of a zigzag topology of chains of monomers, where adjacent gold(I) centres interact aurophilically. However, similar reaction of 1 with KI solution affords the unusual and unexpected compound, [(RNC)2Au][AuI2]
(3). This iodide 3, is a linear chain polymer of alternating anion, cation units that is formed via ligand redistribution. The complexes 1–3 all fluoresce in the presence of UV light; the fluorescence of 3 being most prevalent at room temperature. The free trimethylamine-isocyanoborane ligand, the dimeric structures of compounds 1, 2 and the thermodynamic stabilities of the dimers in the gas phase were studied by density functional and ab initio calculations at various levels of theory. The theoretical results are compared to the corresponding experimental data.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...