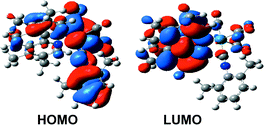
[Re(CO)3(CNx)(L)]+, where CNx = 2,6-dimethylphenylisocyanide, forms complexes with L = 1,10-phenanthroline (1), 4-methyl-1,10-phenanthroline (2), 4,7-dimethyl-1,10-phenanthroline (3), 3,4,7,8-tetramethyl-1,10-phenanthroline (4), 2,9-dimethyl-1,10-phenanthroline (5) and 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (6). The metal–ligand-to-ligand charge transfer transition (MLLCT) absorption bands follow the series: 3
(27 800 cm−1) > 1, 2, 4 and 5
(27 500 cm−1) > 6
(26 600 cm−1). Density functional theory (DFT) geometry optimizations reveal elongated Re–N (L) distances of 2.28 and 2.27 Å for 5 and 6, respectively, compared to 2.23 Å for 1–4. The reversible reduction potentials (E1/2(red)) of 1–4 are linearly dependent on the B3LYP calculated LUMO energies. Time-dependent (TD) DFT and conductor-like polarizable continuum model (CPCM) calculated singlet excited states deviate by 700 cm−1 or less from the experimental absorption maxima and aid in the spectral assignments. The 3MLLCT emitting state energies are within 900 cm−1 of the experimental 77 K emission energies for 1–6. The 77 K emission energies, E1/2(red), and the room temperature emission quantum yields (ϕLUMOem) decrease in the order 1 > 2 > 3 > 4 whereas ELUMO and the room temperature emission energies follow the opposite trend. The emission lifetimes (τem) decrease in the order 3 > 4 > 2 > 1 > 5 with 3 having the highest emission lifetime values of 26.9 µs at room temperature and 384 µs at 77 K and complex 5 having the lowest emission lifetimes of 4.6 µs at room temperature and 61 µs and 77 K.

 Please wait while we load your content...
Please wait while we load your content...