Mononuclear oxovanadium complexes of tris(2-pyridylmethyl)amine†
Abstract
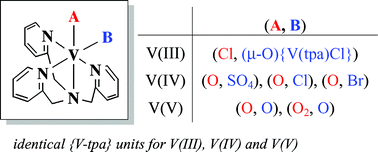
Mononuclear oxovanadium(IV) and dioxovanadium(V) complexes of tris(2-pyridylmethyl)amine (tpa) have been prepared for the first time. Crystal structure determinations of three oxovanadium(IV) complexes, [VO(SO4)(tpa)], [VOCl(tpa)]PF6, or [VOBr(tpa)]PF6, and a dioxovanadium(V) complex [V(O)2(tpa)]PF6 disclosed that the tertiary nitrogen of the tpa ligand always occupies the trans-to-oxo site. The structures of an oxo-peroxo complex [VO(O2)(tpa)]Cl that was prepared previously and of a μ-oxo vanadium(III) complex [{VCl(tpa)}2(μ-O)](PF6)2 have also been determined. The tertiary nitrogen is located at a trans site to the peroxo and chloride ligands, respectively. The total sums of the four V–N bond lengths from the tpa ligand are remarkably similar among the six complexes, indicating that the vanadium oxidation states become less influential in tpa bonding due primarily to the coordination of electron-donating oxo ligand(s). Absorption spectra of [VOCl(tpa)]+ in acetonitrile showed a significant change upon addition of p-toluenesulfonic acid and HClO4, but not on addition of benzoic acid. Protonation at the oxo ligand by the former two acids is suggested. Cyclic voltammetric studies in acetonitrile verified the proton-coupled redox behavior of the VIII/VIV process involving the oxo ligand for the first time. From the dependence of the added p-toluenesulfonic acid to the CV, redox potentials for the following species have been estimated: [VIVOCl(tpa)]+/[VIIIOCl(tpa)] (E1/2 = −1.59 V vs. Fc+/Fc), [VIV(OH)Cl(tpa)]2+/[VIII(OH)Cl(tpa)]+ (Epc = −1.34 V), [VIV(OH2)Cl(tpa)]3+/[VIII(OH2)Cl(tpa)]2+ (Epa = −0.49 V), and [VIVCl2(tpa)]2+/[VIIICl2(tpa)]+ (E1/2 = −0.89 V). The reduction of [VV(O)2(tpa)]+ in 0.05 M [(n-Bu)4N]PF6 acetonitrile showed a major irreversible reduction wave V(V)/(IV) at −1.48 V. The metal reduction potentials of the oxovanadium(IV) and dioxovanadium(V) species are very close, reinforcing the significant influence of the oxo ligand(s).

 Please wait while we load your content...
Please wait while we load your content...