Hydrogen atom vs electron transfer in catecholase-mimetic oxidations by superoxometal complexes. Deuterium kinetic isotope effects†
Abstract
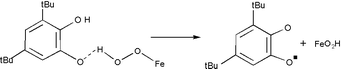
Dioximato-cobalt(II), -iron(II) and -manganese(II) complexes (1)–(6), acting as functional catecholase and phenoxazinone synthase models, exhibit a deuterium kinetic isotope effect predicted by theory (k4H/k4D ≤ 3) in the catalytic oxidative dehydrogenation of 3,5-di-tert-butylcatechol and 2-aminophenol by O2. KIEs in the range of (k4H/k4D ≈ 1.79–3.51) are observed with (1) and (2) as catalysts, pointing to hydrogen atom transfer in the rate-determining step from the substrate hydroxy group to the metal-bound superoxo ligand. Less significant KIEs (1.06–1.20) are exhibited by catalysts systems (3)–(6), indicating that proton-coupled electron transfer is the preferred route in those cases.

 Please wait while we load your content...
Please wait while we load your content...