Ultrafast dynamics of excess electrons in molten salts: Part II.† Femtosecond investigations of Na–NaBr and Na–NaI melts
Abstract
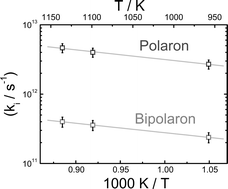
Ultrafast dynamics of excess electrons in Na–NaBr and Na–NaI molten solutions at elevated temperatures (T = 953–1128 K) were investigated over an extended wavelength range. Modelling the time profiles resulted in two time constants τ1 = (200 ± 40) fs and τ2 = (2.8 ± 0.4) ps for both systems at 1073 K. All transients can be understood in terms of dynamical equilibria between polaron and Drude-type electrons as well as polaron and Drude-type electron forming bipolarons. In agreement with our earlier results for K–KCl melts the fast component is assigned to the relaxation of Drude-type electrons into polarons while the longer component, τ2, represents the time during which Drude-type electrons recombine with polarons leading to bipolarons. In addition, the temperature dependence was studied in Na–NaI: Decreasing the temperature to 953 K resulted in an increase of the time constants to τ1 = (360 ± 50) fs and τ2 = (4.3 ± 0.7) ps, respectively. At temperatures, where the ionic diffusion in Na–NaI melts becomes comparable to Na–NaBr melts, the time constants for the relaxation processes also coincide. The temperature-dependent investigations resulted in an Arrhenius activation energy of (25 ± 5) kJ mol−1 for Na–NaI melts in good agreement with literature data.

 Please wait while we load your content...
Please wait while we load your content...