The role of the radical-complex mechanism in the ozone recombination/dissociation reaction
Abstract
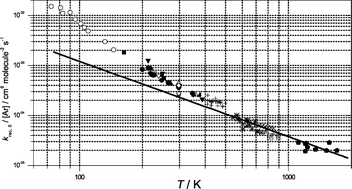
The data bases for low-pressure rate coefficients of the dissociation of O3 and the reverse recombination of O with O2 in the bath gases M = He, Ar, N2, CO2 and SF6 are carefully analyzed. At very high temperatures, the rate constants have to correspond solely to the energy transfer (ET) mechanism. On condition that this holds for Ar and N2 near 800 K, average energies transferred per collision of −〈ΔE〉/hc
= 18 and 25 cm−1 are derived, respectively. Assuming an only weak temperature dependence of 〈ΔE〉 as known in similar systems, rate coefficients for the ET-mechanism are extrapolated to lower temperatures and compared with the experiments. The difference between measured and extrapolated rate coefficients is attributed to the radical complex (RC) mechanism. The derived rate coefficients for the RC-mechanism are rationalized in terms of equilibrium constants for equilibria of van der Waals complexes of O (or O2) with the bath gases and with rate coefficients for oxygen abstraction from these complexes. The latter are of similar magnitude as rate coefficients for oxygen isotope exchange which provides support for the present interpretation of the reaction in terms of a superposition of RC- and ET-mechanisms. We obtained rate coefficients for the ET-mechanism of kETrec,0/[Ar]
= 2.3 × 10−34 (T/300)−1.5 and kETrec,0/[N2]
= 3.5 × 10−34
(T/300)−1.5 and kETrec,0/[N2]
= 3.5 × 10−34 (T/300)−1.5 cm6 molecule−2 s−1 and rate coefficients for the RC-mechanism of kRCrec,0/[Ar]
= 1.7 × 10−34
(T/300)−1.5 cm6 molecule−2 s−1 and rate coefficients for the RC-mechanism of kRCrec,0/[Ar]
= 1.7 × 10−34 (T/300)−3.2 and kRCrec,0/[N2] = 2.5 × 10−34
(T/300)−3.2 and kRCrec,0/[N2] = 2.5 × 10−34 (T/300)−3.3 cm6 molecule−2 s−1. The data bases for M = He, CO2 and SF6 are less complete and only approximate separations of RC- and ET-mechanism were possible. The consequences of the present analysis for an analysis of isotope effects in
(T/300)−3.3 cm6 molecule−2 s−1. The data bases for M = He, CO2 and SF6 are less complete and only approximate separations of RC- and ET-mechanism were possible. The consequences of the present analysis for an analysis of isotope effects in

 Please wait while we load your content...
Please wait while we load your content...