Enhanced photocatalytic formation of hydroxyl radicals on fluorinated TiO2
Abstract
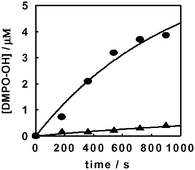
Direct experimental evidence of the higher concentration of hydroxyl radicals generated on fluorinated titanium dioxide (F–TiO2) under irradiation was obtained by spin-trapping EPR measurements. The faster photoinduced bleaching of the azo dye Acid Red 1 (AR1) observed in the presence of F–TiO2 was explained by the high affinity of the azo double bond towards ˙OH radicals. Moreover, the pronounced decrease of the AR1 bleaching rate by addition of 2-propanol, as hydroxyl radicals scavenger, on F–TiO2 and not on naked TiO2 demonstrated that on fluorinated titania AR1 is mainly degraded via ˙OH radical attack.

 Please wait while we load your content...
Please wait while we load your content...