Enantiomerically pure P-chiral phosphinoselenoic chlorides: inversion of configuration at the P-chirogenic center in the synthesis and reaction of these substances†
Abstract
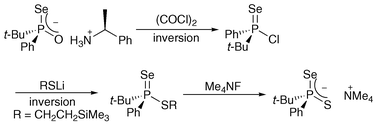
Reaction of diastereomerically pure phosphinoselenoic acid salts with
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST

 Please wait while we load your content...
Please wait while we load your content...