Carborane acids. New “strong yet gentle” acids for organic and inorganic chemistry
Abstract
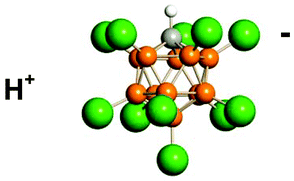
Icosahedral carborane anions such as CHB11Cl11− are amongst the least coordinating, most chemically inert anions known. They are also amongst the least basic, so their conjugate acids, H(carborane), are superacids (i.e. stronger than 100% H2SO4). Acidity scale measurements indicate that H(CHB11Cl11) is the strongest pure Brønsted acid presently known, surpassing triflic and ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C+–R), silylium ions (R3Si+) and discrete hydronium ions (H3O+, H5O2+etc.) can be readily isolated as
C+–R), silylium ions (R3Si+) and discrete hydronium ions (H3O+, H5O2+etc.) can be readily isolated as

 Please wait while we load your content...
Please wait while we load your content...