Analytical chemistry of synthetic routes to psychoactive tryptamines
Part II.† Characterisation of the Speeter and Anthony synthetic route to N,N-dialkylated tryptamines using GC-EI-ITMS, ESI-TQ-MS-MS and NMR‡
Abstract
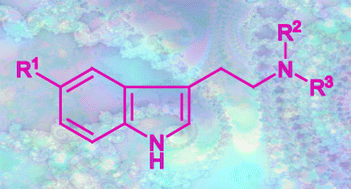
The degree of alkylation of the side chain nitrogen in tryptamines is one important factor that affects psychoactivity. The method of Speeter and Anthony is considered to be one of the most important synthetic preparative methods. The final step in this reaction is based on the reduction of a (substituted) indole-3-yl-glyoxalylamide to the desired tryptamine with metal hydride. Twelve symmetrically and 13 asymmetrically N,N-disubstituted glyoxalylamides and their corresponding tryptamine derivatives have been synthesised and characterised by gas chromatography EI-ion trap mass spectrometry, electrospray-triple quadrupole-tandem mass spectrometry and NMR spectroscopy. Mass spectral and NMR similarities and differences between the investigated compounds are discussed. A solvent dependency is observed that has to be taken into consideration for the unambiguous assignment of 1H- and 13C-NMR chemical shifts. The 1H-NMR study demonstrated that one can evaluate the rotamer populations of the asymmetrical glyoxalylamides. In a forensic or clinical scenario where single or multiple reaction monitoring approaches are contemplated, the appropriate ion transitions of choice may then focus on the two main fragmentations, namely β-cleavage ([M+H]+ → CH2N+R2R3) and/or α-cleavage ([M+H]+ → [3-vinylindole]+), respectively. The synthesis, NMR and MS analytical data presented provide the forensic analyst and clinical biochemist with a detailed and self-consistent body of information and mechanisms for the spectral identification of the more likely psychoactive tryptamines that may be met.

 Please wait while we load your content...
Please wait while we load your content...