Synthesis and stability of exocyclic triazine nucleosides
Abstract
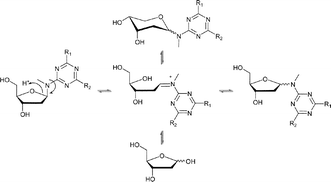
Synthesis and stability studies of exocyclic amino triazine nucleosides were performed. Stability of the nucleosides was found to be dependent on triazine ring electron density, solvent, and pH. The nucleosides were found to be more stable when the triazine ring was electron deficient, in high pH aqueous solutions and in aprotic solvents.

 Please wait while we load your content...
Please wait while we load your content...